Introduction
The development of targeted cancer therapies has significantly transformed modern oncology, offering more precise treatment options with improved outcomes and reduced toxicity compared to traditional chemotherapy. Among these targeted therapies, Cyclin-Dependent Kinase 4 and 6 (CDK4/6) inhibitors represent one of the most important breakthroughs in the management of hormone receptor–positive breast cancer and other malignancies. Here, we will explore the CDK4/6 inhibitor drug class in detail, including its biological rationale, mechanism of action, approved agents, clinical applications, safety profile, resistance mechanisms and emerging future directions.
Definition
CDK4/6 inhibitors are a class of targeted anticancer drugs that selectively inhibit cyclin-dependent kinases 4 and 6 (CDK4 and CDK6), enzymes that regulate progression of the cell cycle from the G1 phase to the S phase. By blocking CDK4/6 activity, these agents prevent phosphorylation of the retinoblastoma (Rb) protein, leading to cell-cycle arrest and suppression of tumor cell proliferation. CDK4/6 inhibitors are primarily used in the treatment of hormone receptor–positive, HER2-negative breast cancer and are often administered in combination with endocrine therapy. Common drugs in this class include palbociclib, ribociclib, and abemaciclib.
Understanding the Cell Cycle and the Role of CDK4/6
The cell cycle is a tightly regulated process that governs cell growth, DNA replication, and cell division. It consists of several phases – G1, S, G2, and M – each controlled by specific molecular checkpoints. Dysregulation of the cell cycle is a hallmark of cancer, leading to uncontrolled cell proliferation.
Cyclin-dependent kinases (CDKs) are a family of enzymes that play a central role in regulating the cell cycle. Among them, CDK4 and CDK6, when complexed with cyclin D, drive the transition from the G1 phase to the S phase, where DNA synthesis occurs. This transition is primarily mediated through phosphorylation of the retinoblastoma (Rb) protein, a tumor suppressor that normally inhibits cell cycle progression.
In many cancers – particularly hormone receptor–positive breast cancer – this CDK4/6–cyclin D–Rb pathway is overactivated, resulting in unchecked cell division. CDK4/6 inhibitors were specifically designed to interrupt this pathway and restore cell cycle control.
Mechanism of Action of CDK4/6 Inhibitors
CDK4/6 inhibitors work by selectively inhibiting CDK4 and CDK6 enzymatic activity, preventing phosphorylation of the Rb protein. When Rb remains in its hypophosphorylated (active) state, it continues to suppress E2F transcription factors, thereby halting progression from the G1 to the S phase.
This results in cell cycle arrest, rather than direct cell death. Cancer cells are prevented from proliferating, making them more susceptible to hormonal therapies or other anticancer agents. Importantly, CDK4/6 inhibitors demonstrate selectivity for cancer cells that rely heavily on this pathway, helping to spare normal cells and reduce systemic toxicity.
Approved CDK4/6 Inhibitors
Currently, three CDK4/6 inhibitors have gained regulatory approval and are widely used in clinical practice:
1. Palbociclib
Palbociclib was the first CDK4/6 inhibitor approved for clinical use. It is commonly used in combination with endocrine therapies such as aromatase inhibitors or fulvestrant. Palbociclib is known for its effectiveness but is associated with higher rates of neutropenia compared to other agents in this class.
2. Ribociclib
Ribociclib has demonstrated strong efficacy in improving overall survival in patients with advanced hormone receptor–positive breast cancer. It shares a similar safety profile with palbociclib but requires monitoring for potential QT interval prolongation and liver enzyme elevations.
3. Abemaciclib
Abemaciclib is more selective for CDK4 than CDK6 and can be used either in combination with endocrine therapy or as monotherapy. Unlike the other two agents, it causes less neutropenia but more gastrointestinal side effects, particularly diarrhea.
Clinical Applications
Hormone Receptor–Positive Breast Cancer
The primary indication for CDK4/6 inhibitors is estrogen receptor–positive (ER+), HER2-negative advanced or metastatic breast cancer. When combined with endocrine therapy, these drugs have shown:
- Significant improvement in progression-free survival
- Enhanced overall survival
- Delayed onset of chemotherapy
- Improved quality of life for patients
CDK4/6 inhibitors are now considered standard of care in both first-line and subsequent treatment settings for this patient population.
Early-Stage Breast Cancer
Recent studies have expanded the use of CDK4/6 inhibitors into early-stage, high-risk breast cancer. Abemaciclib, in particular, has been approved as adjuvant therapy for certain high-risk patients, marking a major advancement in preventing cancer recurrence.
Investigational Uses in Other Cancers
Beyond breast cancer, CDK4/6 inhibitors are being studied in several other malignancies, including:
- Lung cancer
- Melanoma
- Glioblastoma
- Liposarcoma
- Colorectal cancer
While results have been mixed, ongoing clinical trials continue to explore combination strategies that may broaden their therapeutic impact.
Safety Profile and Adverse Effects
Although generally well tolerated, CDK4/6 inhibitors are associated with predictable side effects due to their impact on proliferating cells.
Common Adverse Effects
- Neutropenia
- Anemia and thrombocytopenia
- Fatigue
- Nausea
- Diarrhea (especially with abemaciclib)
Monitoring Requirements
- Regular complete blood counts (CBC)
- Liver function tests
- Electrocardiogram monitoring (for ribociclib)
Dose adjustments and treatment interruptions are often effective in managing toxicities without compromising efficacy.
Resistance to CDK4/6 Inhibitors
Despite their success, resistance to CDK4/6 inhibitors eventually develops in many patients. Known resistance mechanisms include:
- Loss or mutation of the Rb protein
- Amplification of cyclin E
- Activation of alternative signaling pathways (e.g., PI3K/AKT/mTOR)
- Upregulation of CDK2 activity
Understanding these mechanisms has fueled research into rational drug combinations designed to overcome resistance.
Combination Strategies and Future Directions
The future of CDK4/6 inhibitors lies in combination therapy and precision medicine. Current research focuses on combining CDK4/6 inhibitors with:
- PI3K inhibitors
- mTOR inhibitors
- Immune checkpoint inhibitors
- Novel endocrine agents
Biomarker development is also a major area of interest, aiming to identify which patients are most likely to benefit from treatment and to predict resistance early.
Additionally, next-generation CDK inhibitors with broader or more selective activity are under investigation, potentially expanding the scope of cell cycle–targeted therapies beyond CDK4/6.
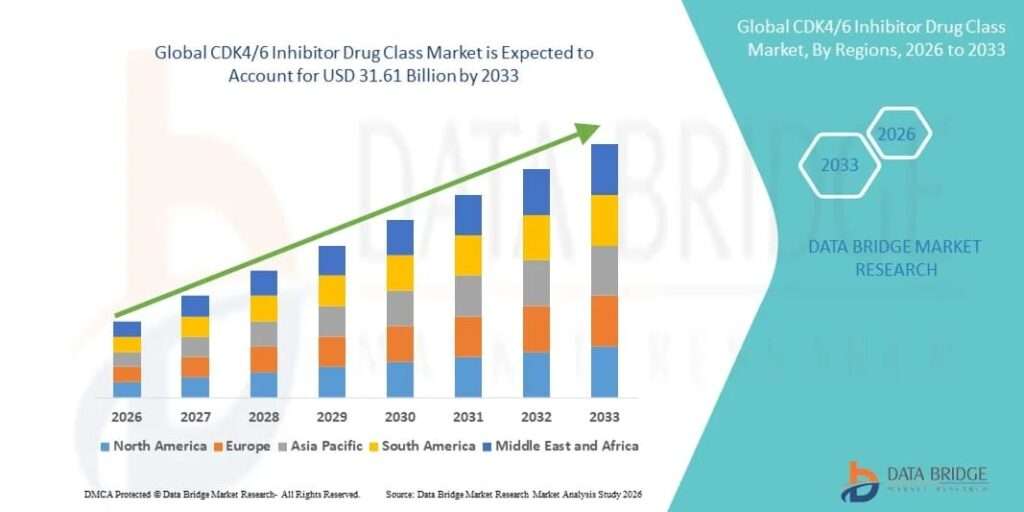
Growth Rate of CDK4/6 Inhibitor Drug Class Market
According to Data Bridge Market Research, the CDK4/6 inhibitor drug class market was estimated to be worth USD 15.52 billion in 2025 and is projected to grow at a compound annual growth rate (CAGR) of 9.30% to reach USD 31.61 billion by 2033.
Learn More: https://www.databridgemarketresearch.com/reports/global-cdk46-inhibitor-drug-class-market
Conclusion
The CDK4/6 inhibitor drug class has revolutionized the treatment landscape for hormone receptor–positive breast cancer, offering substantial improvements in survival and quality of life. By targeting a fundamental mechanism of cancer cell proliferation, these agents exemplify the power of rational drug design and precision oncology.