Introduction
Antibiotics are among the most important medical discoveries in human history. Since the discovery of penicillin in the early 20th century, antibiotics have saved millions of lives by effectively treating bacterial infections that were once fatal. Behind every antibiotic tablet, capsule, or injection lies a complex and carefully controlled production process. Antibiotic production combines microbiology, biochemistry, chemical engineering, and strict quality control to ensure safety and effectiveness. Here, we explore how antibiotics are produced, the key methods involved, the challenges faced by manufacturers, and the future of antibiotic production.
Definition
Antibiotic production is the process of manufacturing substances that inhibit the growth of or destroy harmful microorganisms, especially bacteria. It typically involves cultivating microorganisms such as bacteria or fungi under controlled conditions, allowing them to produce antibiotic compounds, which are then extracted, purified, and formulated for medical use to prevent or treat infectious diseases.
What Are Antibiotics?
Antibiotics are substances that either kill bacteria (bactericidal) or inhibit their growth (bacteriostatic). They work by targeting specific bacterial structures or processes, such as cell wall synthesis, protein production, or DNA replication. Antibiotics can be classified into several groups, including penicillins, cephalosporins, tetracyclines, macrolides, and aminoglycosides, each with distinct mechanisms of action.
Most antibiotics are either produced naturally by microorganisms or derived from natural compounds through chemical modification. A smaller number are fully synthetic.
Sources of Antibiotics
The primary sources of antibiotics are microorganisms, particularly:
- Bacteria (e.g., Streptomyces species)
- Fungi (e.g., Penicillium and Cephalosporium)
- Actinomycetes, which are filamentous bacteria responsible for producing many clinically important antibiotics
These microorganisms naturally produce antibiotics as a survival mechanism to compete with other microbes in their environment. Scientists harness this natural ability on an industrial scale.
Methods of Antibiotic Production
Antibiotic production generally involves three main methods:
1. Fermentation Process
Fermentation is the most common and important method for producing antibiotics. In this process, selected microorganisms are grown in large fermentation tanks (bioreactors) under controlled conditions.
Key steps include:
- Selection of microorganism: A high-yield, stable strain is chosen or genetically improved.
- Preparation of growth medium: The medium contains carbon sources (such as glucose), nitrogen sources, minerals, and vitamins.
- Sterilization: All equipment and media are sterilized to prevent contamination.
- Fermentation: Microorganisms are cultured at optimal temperature, pH, oxygen level, and agitation speed.
- Production phase: Antibiotics are usually produced during the stationary phase of microbial growth.
Fermentation can be submerged fermentation (liquid medium) or solid-state fermentation, depending on the antibiotic.
2. Isolation and Purification
Once fermentation is complete, the antibiotic must be separated from the fermentation broth. This step is crucial for ensuring purity and safety.
Common techniques include:
- Filtration or centrifugation to remove microbial cells
- Solvent extraction
- Precipitation
- Chromatography
Purification ensures that impurities, toxins, and unwanted by-products are removed.
3. Chemical Modification and Formulation
Some antibiotics undergo chemical modification to improve their effectiveness, stability, or resistance to bacterial enzymes. These are known as semi-synthetic antibiotics, such as amoxicillin.
After purification, antibiotics are formulated into suitable dosage forms like tablets, capsules, syrups, or injectable solutions. Excipients are added to improve stability, absorption, and shelf life.
Quality Control in Antibiotic Production
Antibiotic production is subject to strict regulatory standards due to its impact on human health. Quality control tests are performed at every stage of production.
These include:
- Microbiological assays to measure antibiotic potency
- Chemical analysis to verify purity and composition
- Sterility testing
- Stability testing
- Toxicity and safety assessments
Manufacturers must comply with Good Manufacturing Practices (GMP) to ensure consistent product quality.
Challenges in Antibiotic Production
Despite its success, antibiotic production faces several challenges:
Antibiotic Resistance:
One of the biggest global health threats is antibiotic resistance. Overuse and misuse of antibiotics have led to the emergence of resistant bacteria, reducing the effectiveness of existing drugs. This creates pressure on manufacturers to develop new antibiotics, which is costly and time-consuming.
High Production Costs:
Antibiotic production requires expensive infrastructure, skilled personnel, and strict regulatory compliance. Maintaining sterile conditions and quality control adds to operational costs.
Environmental Concerns:
Improper disposal of antibiotic waste can contaminate soil and water, contributing to environmental resistance. Manufacturers must invest in waste treatment and sustainable practices.
Limited Discovery of New Antibiotics:
The discovery of novel antibiotic classes has slowed significantly. Many pharmaceutical companies have reduced investment in antibiotic research due to lower profitability compared to chronic disease drugs.
Advances and Future Trends
The future of antibiotic production depends on innovation and sustainability. Several promising developments are shaping the field:
Biotechnology and Genetic Engineering:
Genetic modification of microorganisms can significantly increase antibiotic yield and efficiency. Techniques such as CRISPR and metabolic engineering allow scientists to optimize production pathways.
Synthetic Biology:
Synthetic biology enables the design of new biological systems to produce novel antibiotics or improve existing ones. This approach may help overcome resistance.
Green and Sustainable Production:
Efforts are being made to reduce environmental impact by using eco-friendly solvents, renewable raw materials, and energy-efficient processes.
Artificial Intelligence in Drug Discovery:
AI and machine learning are being used to identify new antibiotic candidates and optimize fermentation conditions, reducing development time.
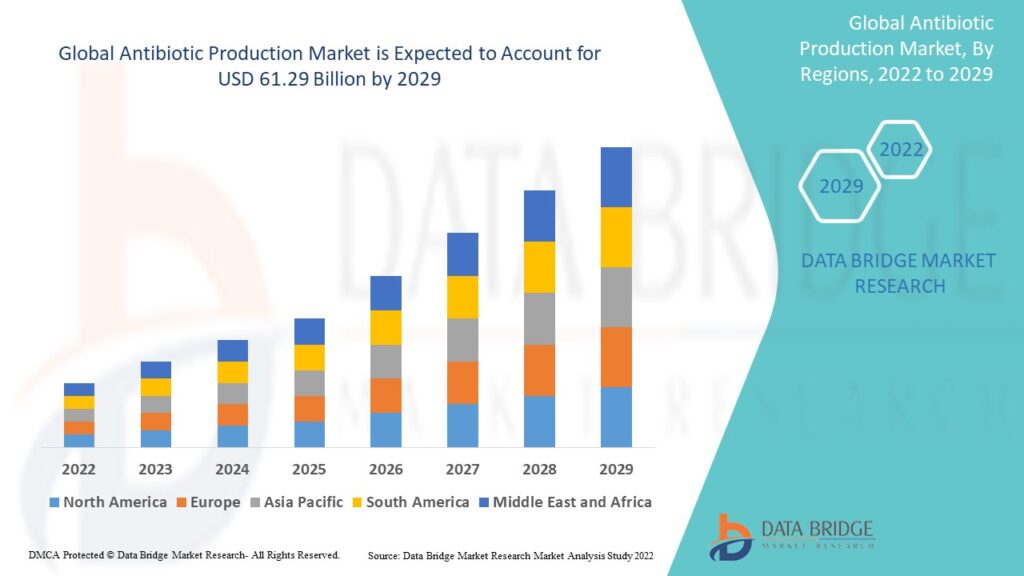
Growth Rate of Antibiotic Production Market
According to Data Bridge Market Research, the size of the global antibiotic production market was estimated at USD 47.45 billion in 2024 and is expected to grow at a compound annual growth rate (CAGR) of 5.25% from 2025 to 2032, reaching USD 71.46 billion.
Learn More: https://www.databridgemarketresearch.com/reports/global-antibiotic-production-market
Conclusion
Antibiotic production is a vital and complex process that plays a crucial role in modern healthcare. From microbial fermentation to purification and formulation, each step requires precision, expertise, and strict quality control. While challenges such as antibiotic resistance and high production costs persist, advances in biotechnology and sustainable practices offer hope for the future. Continued investment in research, responsible antibiotic use, and innovative production methods are essential to ensure that antibiotics remain effective tools in fighting infectious diseases for generations to come.